What is NMR? What is Spectrometer? What is Chemical shift?
Introduction
NMR spectroscopy is the most powerful tool available for organic structure determination.It is used to study a wide variety of nuclei.
The most common type of NMR: Proton and Carbon-13
1H NMR (PMR): To determine the type and number of carbon of H atoms and spatial arrangement of them in a molecule.
13C NMR Nuclear Magnetic Resonance(NMR) is a physical phenomenon in which nuclei in a magnetic field absorb and re-emit electromagnetic radiation.
(CMR): To determine the type and number of carbon atoms and spatial arrangement of them in a molecule.
The source of energy in NMR is radio waves which have long wavelengths (75-0.5m), and thus low energy and frequency (4-600MHz).
Principles of NMR
The theory behind NMR comes from a spin of a nucleus and it generates a magnetic field. Without an external applied magnetic field, the nuclear spins are random in directions. But when an external magnetic field (H0), is present the nuclei align themselves either with or against the field of the external magnet.The spin of a nucleus
Magnetic and nonmagnetic Nuclei
To find out nuclear spin quantum no. 1 Following rules are generally applied.A nucleus with I>0 when spins about its own axis generate a magnetic moment and are known as magnetic nuclei.
The nucleus with I=0 does not produce magnetic moment are known as nonmagnetic nuclei The nuclei which have even mass number and even atomic numbers have zero spin quantum number are nonmagnetic.
The nuclei with I>0 possesses spin angular momentum and produce a magnetic field are known as magnetic nuclei.
- The which have odd mass no. and odd or even atomic no. have half-integral spin like ½, 3/2,5/2, and possesses spin quantum no. I=½. These are magnetic nuclei.
- The nuclei having even mass no. and odd atomic no. have integral spin no.such as 1,2,3, etc possesses spin quantum no.I=1. These are also magnetic nuclei.
The no. of orientations of the spin state is given by (2I+1) where I is the spin quantum no. We know that proton(H) has I=½ and it has only two orientations.
The energy required for flipping depends upon the strength of the applied magnetic field.
Nuclear Resonance
NMR Spectrometer
NMR Spectrometer
Different nuclei absorb Electromagnetic radiation at a different wavelength (energy required to bring about resonance). Nuclei of a given type will resonate at different energies depending on their chemical and electronic environment. The position (chemical shift) and pattern (splitting or multiplicity) of NMR signals give important information about the chemical environment of the nuclei.
Chemical shift
The magnetic field at the nucleus is not equal to the applied magnetic field; electrons around the nucleus shield it from the applied field. The difference between the applied magnetic field and the field at the nucleus is termed the nuclear shielding.
Consider the s-electrons in a molecule. They have spherical symmetry and circulate in the applied field, producing a magnetic field which opposes the applied field. This means that the applied field strength must be increased for the nucleus to absorb at its transition frequency. This upfield shift is also termed a diamagnetic shift.
Electrons in p-orbitals have no spherical symmetry. They produce comparatively large magnetic fields at the nucleus, which give a low field shift. This "deshielding" is termed a paramagnetic shift.
In proton (1H) NMR, p-orbitals play no part (there aren't any!), which is why only a small range of chemical shifts (10 ppm) is observed. We can easily see the effect of s-electrons on the chemical shift by looking at substituted methanes, CH3X. As X becomes increasingly electronegative, so the electron density around the protons decreases, and they resonate at lower field strengths (increasing dH values).
Chemical shift is defined as nuclear shielding / applied magnetic field. Chemical shift is a function of the nucleus and its environment. It is measured relative to a reference compound. For 1H NMR, the reference is usually tetramethylsilane, Si (CH3)4.
Spin - spin coupling
Consider the structure of ethanol;
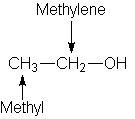
The 1H NMR spectrum of ethanol (below) shows the methyl peak has been split into three peaks (a triplet) and the methylene peak has been split into four peaks (a quartet). This occurs because there is a small interaction (coupling) between the two groups of protons. The spacings between the peaks of the methyl triplet are equal to the spacings between the peaks of the methylene quartet. This spacing is measured in Hertz and is called the coupling constant, J.
To see why the methyl peak is split into a triplet, let's look at the methylene protons. There are two of them, and each can have one of two possible orientations (aligned with or opposed against the applied field). This gives a total of four possible states;
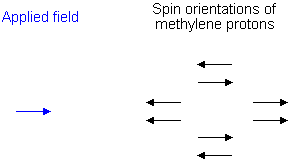
In the first possible combination, spins are paired and opposed to the field. This has the effect of reducing the field experienced by the methyl protons; therefore a slightly higher field is needed to bring them to resonance, resulting in an upfield shift. Neither combination of spins opposed to each other has an effect on the methyl peak. The spins paired in the direction of the field produce a downfield shift. Hence, the methyl peak is split into three, with the ratio of areas 1:2:1.
Similarly, the effect of the methyl protons on the methylene protons is such that there are eight possible spin combinations for the three methyl protons;
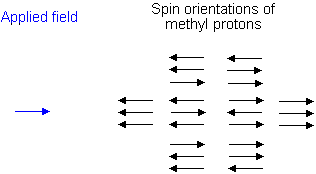
Out of these eight groups, there are two groups of three magnetically equivalent combinations. The methylene peak is split into a quartet. The areas of the peaks in the quartet have the ration 1:3:3:1.
In a first-order spectrum (where the chemical shift between interacting groups is much larger than their coupling constant), interpretation of splitting patterns is quite straightforward;
- The multiplicity of a multiplet is given by the number of equivalent protons in neighboring atoms plus one, i.e. the n + 1 rule
- Equivalent nuclei do not interact with each other. The three methyl protons in ethanol cause splitting of the neighboring methylene protons; they do not cause splitting among themselves
- The coupling constant is not dependant on the applied field. Multiplets can be easily distinguished from closely spaced chemical shift peaks.




Comments
Post a Comment